Regulatory & Quality Assurance Services for the Medical Devices Industry
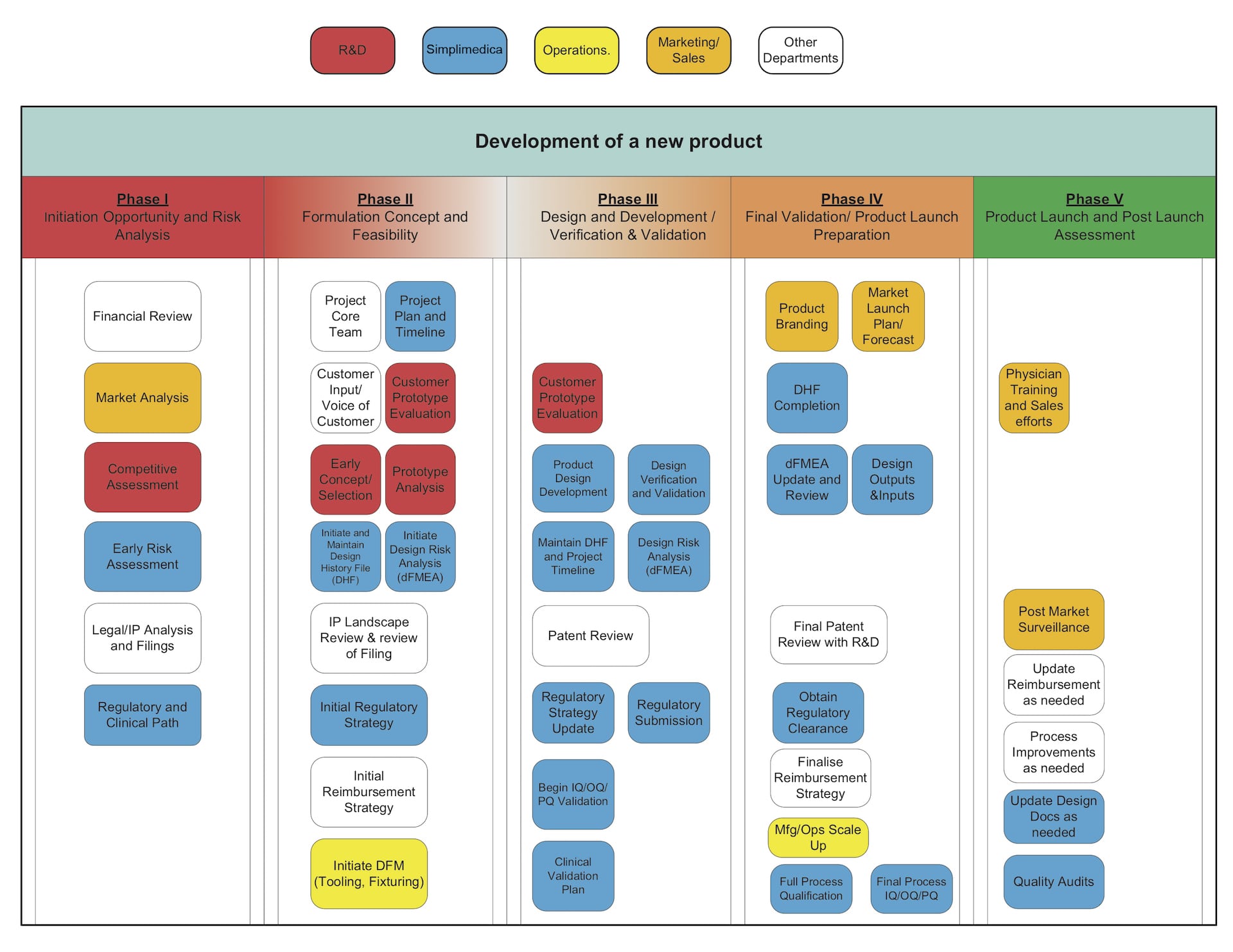
Concept to Launch > Ideas to Reality
Simplimedica supports short-term, medium-term, and long-term project work for small and medium-sized businesses, as well as large corporations that may have a shortage in a skill or wish to outsource work to improve productivity.
We help companies establish quality management systems (QMS) compliant with medical device industry standards. We also provide guidance where regulatory requirements can be overwhelming and confusing. Simplimedica is a one-stop solution for ensuring that your company’s medical devices are firmly on track for being approved for sale in target markets.
Ensure medical devices meet the required high standards
Guide clients to meet regulatory requirements