Turning an idea into a thriving business takes expertise, strategy, and connections. At Simplimedica, we provide medical device startups with the tools and insights they need to successfully navigate market entry, secure funding, and achieve commercial success.

Gain a deep understanding of your target market and competition with our expert insights.
Assess market potential, adoption rates, and revenue opportunities.
Identify key decision-makers from healthcare providers and hospital procurement teams to investors and payers.
Analyse the competitive landscape, pricing strategies, and key differentiators.
Develop a roadmap for entry and expansion based on healthcare infrastructure and funding mechanisms.
Identify the best trial locations and research institutions in the UK, Gulf Cooperation Council (GCC), and USA.
Support post-market studies to enhance adoption and payer engagement.

25+ Years of Regulatory Experience

Let’s turn your vision into reality. Contact Simplimedica today!
Simplimedica will write a Regulatory Roadmap, which typically includes ensuring the device fits the definition of a Medical Device and then looking at the classification of the device in various geographical locations.
Based on this, a strategy is developed to enter those markets with an estimated timeline to entry with estimated costs and a description of the regulatory requirements to overcome those hurdles. The regulatory roadmap serves as a key document for potential investors and shareholders for private funding where there is clarity on the company’s roadmap to success. This provides the potential investors with the confidence they need to invest.
Simplimedica will start with the intended use statement being as clear and precise as possible at the outset to then lay the foundations for market access.
For further details on what a Regulatory Roadmap includes see here.
Secure grants and alternative funding to fuel innovation and commercialization.
Expertise in Innovate UK, NIHR, Horizon Europe, and Biomedical Catalyst applications.
Support for NIH, NSF, BARDA, and SBIR/STTR grants.
Identify regional government-backed funding opportunities.
Craft and submit competitive grant applications tailored to funder priorities.
Ensure compliance, reporting, and proper fund utilization.
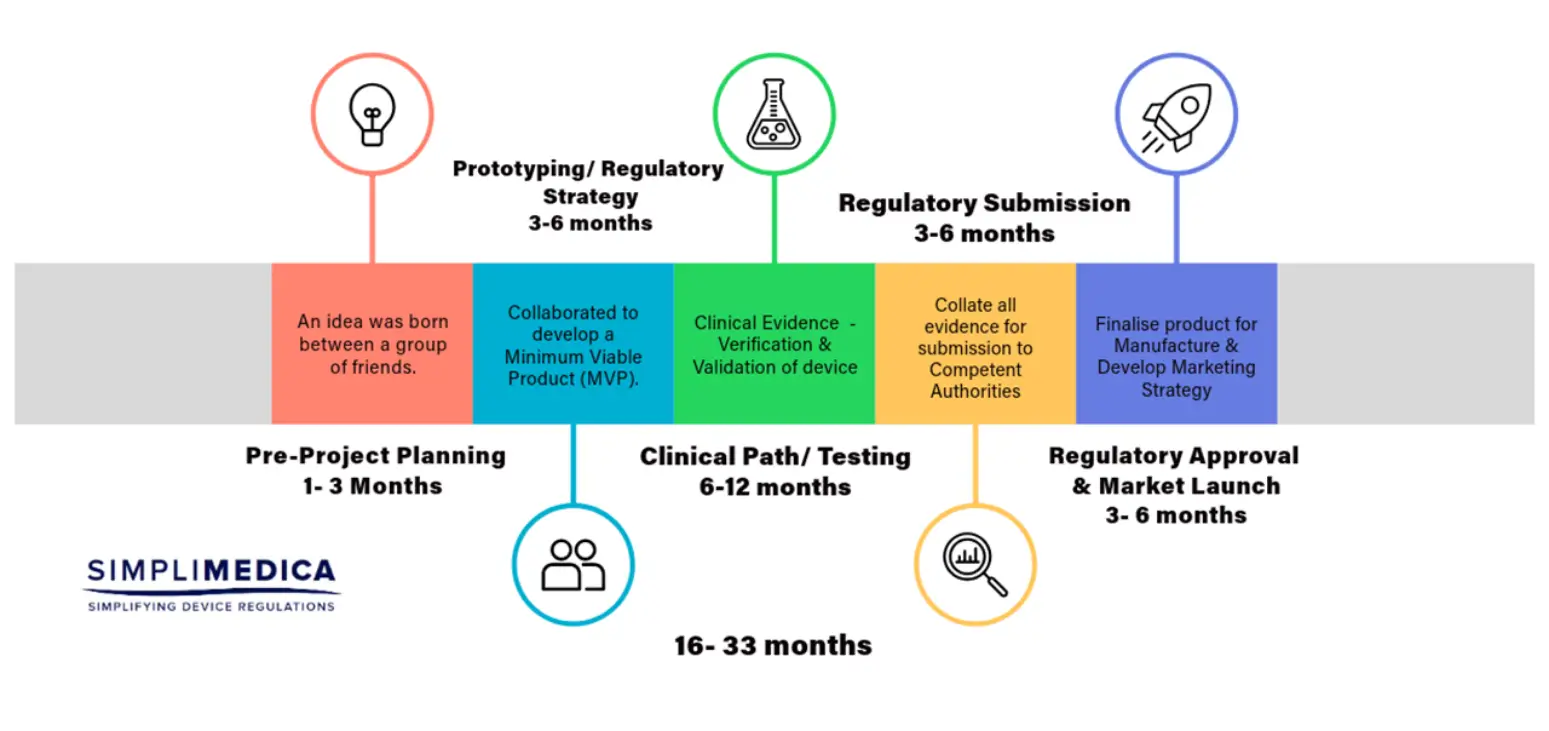

Pre-Seed Stage (1–2 years)
Seed Stage (1–2 years)
